Blog Details

- 21 May 22
How to Prevent Corrosion in TMT Bars?
What is Corrosion?
Corrosion is the process in which a material deteriorates as a result of its interaction with its environment. Corrosion is the primary cause of metal deterioration. It occurs in refined metals as they try to return to their stable form. Many metals or alloys undergo corrosion from exposure to water or air. However, this process can also be facilitated by exposure to other substances.
5 Ways to Prevent Corrosion on TMT Bars
1. Avoid Exposure to Corrosive Agents
Prevent the deterioration of metals by limiting their contact with corrosive agents. For instance, safeguard the metal materials from rainwater or excessive moisture by properly storing them indoors. Moreover, exposure to substances containing chloride (such as saltwater) must be limited. Treat feed water in boilers with softeners to prevent corrosion.
2. Proper Monitoring of Metal Surface
Carefully monitor the metal surface. Look for cracks and crevices, as these flaws can also lead to corrosion. Additionally, use corrosion-resistant products. For example, if you're buying TMT bars for construction, choose corrosion-resistant bars to ensure the longevity of the structure.
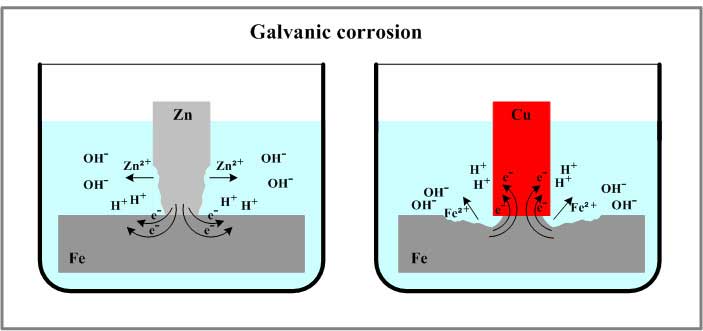
3. Prevent Galvanic Corrosion
When two different metals come into contact in an electrolyte, galvanic corrosion can occur. One metal acts as the anode and corrodes more quickly, while the other acts as the cathode and corrodes more slowly. This is common in pipelines, ship hulls, and boats. To prevent this, apply protective coatings or provide cathodic protection using a sacrificial anode metal.
4. Protect the Metal Surface

Paints form a protective barrier between the metal surface and corrosive agents. For example, coating outdoor metal units with paint protects them from rain or snow. Other solutions include galvanized zinc coating, oil sealants, or specialized paints that reduce exposure to corrosive elements.
5. Metallic Plating

Metallic plating methods such as electroplating, mechanical plating, electroless coating, or hot dipping offer protection. These involve covering the surface with a layer of metal (like zinc, nickel, or cadmium) to prevent corrosion and extend the life of the TMT bars.
Search
Recent Posts

6 Essential Tips for Choosing High-Quality TMT Bars in India
Build stronger with the best quality TMT bars in India. Explore tips to identify the best sariya in India and reliable steel bars for construction.
 29 Jan 26
29 Jan 26

Top 5 Benefits of Corrosion-Resistant TMT Bars in Construction
Protect buildings from rust with Corrosion Resistant TMT Bars. Know the best iron rods for construction, 12 mm saria price per kg & top TMT bar company in India.
 26 Jan 26
26 Jan 26

Top TMT Bar in India: Latest TMT Bar Rates Explained
Explore top TMT steel in India with updated TMT bar rates. Check 10mm, 12mm & 16mm saria price per kg and choose the best steel for construction.
 31 Dec 25
31 Dec 25

Top 10 TMT Bar in India: Best Steel for House Construction
Explore the top 10 TMT bar in India with SRMB. Learn why it’s among the best TMT bar companies offering best quality steel bars for house construction.
 28 Dec 25
28 Dec 25

What Factors Affects TMT Bars Price Per Kg?
Understand the major factors that influence the price per kg of TMT bars, including technology, energy costs, market competition, brand reputation, and seasonal effects. Make informed buying decisions for your construction projects.
 24 May 25
24 May 25

TMT Bar Online: The 6 Growing Trends of Online Purchasing
Buying TMT bars online is transforming India’s construction industry with benefits like time savings, transparent pricing, and wide product variety. It offers secure payments, doorstep delivery, and better deals, making it a smarter choice for both small and large buyers.
 22 May 25
22 May 25

How to determine Genuine Saria TMT Bars available in the Market?
When buying TMT Saria for construction, ensuring quality is crucial for safety and durability. Look for ISI marks, brand logos, genuine surface patterns, reliable customer reviews, and reasonable pricing to identify authentic bars. Always choose trusted suppliers to avoid substandard or fake products.
 17 May 25
17 May 25

How Raw Material Prices Influence TMT Bar Price in India
The TMT industry in India is vital to infrastructure development, with prices heavily influenced by raw material costs like iron ore, scrap steel, and coal. Fluctuations in these materials directly impact TMT bar rates, affecting builders and buyers across the country. Understanding these cost drivers helps make informed purchasing decisions.
 15 May 25
15 May 25

10 Factors Affecting TMT Bar Price in India in 2025
TMT Bar prices in India fluctuate due to factors like raw material costs, transportation, weather, demand-supply balance, and government policies. Economic growth, foreign trade, currency exchange rates, and seasonal trends also play key roles in shaping daily price variations for TMT bars.
 13 May 25
13 May 25

7 Must-Run Quality Tests for Best TMT Bars in India
To ensure strength, flexibility, and durability, the best TMT bars in India undergo seven critical quality tests—Bend, Rust, Dimension, Section Weight, Heat, Tensile, and Chemical. These tests confirm structural integrity, corrosion resistance, and compliance with national standards for safe construction.
 12 May 25
12 May 25

Top 10 Policies Shaping the TMT Bar Company in India
India's TMT steel bar industry is rapidly evolving, driven by key government policies promoting energy efficiency, quality control, digital manufacturing, and sustainable practices. Supportive measures like import-export incentives, recycling mandates, and price regulations are helping the sector become globally competitive and environmentally responsible.
 28 April 25
28 April 25

TMT Saria Price: 5 Key Factors that Influence the Price
Choosing the right TMT Saria is crucial for strong, durable construction. This guide helps you compare supplier prices effectively by considering key factors like tariffs, transport costs, quality, and bulk discounts—ensuring the best value without compromising on quality.
 24 April 25
24 April 25

Iron Rod Price Insights: How to Find the Best Deals
Iron rods are essential for strong, stable concrete structures, making them a core material in construction. Their prices fluctuate due to factors like raw material costs, demand, and government policies. Knowing these factors helps buyers make smarter, cost-effective purchasing decisions.
 24 April 25
24 April 25

Top TMT Bar in India: 10 Key Roles in Modern Construction
TMT bars are the backbone of modern construction, known for their strength, flexibility, and durability. From heat and corrosion resistance to seismic stability, they play a critical role in building safe, long-lasting structures across residential and infrastructure projects.
 20 April 25
20 April 25

TMT bars in India: Past, Present, and Emerging Future Trends
TMT bars have transformed India's construction industry with their unmatched strength, durability, and corrosion resistance. This blog explores their evolution from the 1980s to today, highlighting key innovations, manufacturing trends, and their vital role in future infrastructure development.
 09 April 25
09 April 25

Raw Material Surge: Understanding TMT Bars Price Per Kg
TMT bar prices in India are heavily influenced by raw materials like iron ore, coal, and scrap steel. This blog explores how these cost drivers impact the TMT bar price per kg and highlights the vital role the TMT industry plays in infrastructure growth, economic development, and sustainability.
 02 April 25
02 April 25

Pricing of Best Saria in India: Factors That Influence Its Cost
The price of TMT Saria in India is influenced by factors like raw material costs, energy prices, and market demand. This blog explains what drives TMT Saria pricing and how to choose the best quality bars for long-term value and construction durability.
 28 March 25
28 March 25

The Future of TMT Saria: Trends to Watch in the Steel Industry
The TMT Saria industry is heading into a tech-driven future with innovations like automation, smart sensors, and eco-friendly steel transforming production and performance. With rising demand, sustainability goals, and global expansion, the next decade promises stronger, smarter, and more efficient construction materials for India and beyond.
 28 March 25
28 March 25

Common Mistakes in Using TMT Saria Resources and How to Avoid Them
Using TMT Saria correctly is crucial to ensure structural strength and safety. This blog outlines common mistakes—like choosing the wrong grade, improper storage, or incorrect bending—and offers practical tips to help you avoid them for a durable and cost-effective construction project.
 28 March 25
28 March 25

2025's Perfect Blend: Vastu Shastra & TMT Bars for Strength
In 2025, combining Vastu Shastra’s ancient wisdom with the strength and sustainability of TMT bars is shaping a new era of construction. This fusion ensures buildings that are not only structurally durable but also energetically harmonious, promoting longevity, resilience, and well-being.
 28 March 25
28 March 25

From Blueprint into Reality: Crafting Your Dream Space
Starting a construction project is exciting but demands smart planning, quality materials, and the right team. TMT bars play a crucial role by offering strength, durability, and sustainability, making them essential for building safe, long-lasting structures that meet modern needs.
 28 March 25
28 March 25

Construction Industry Trends to Watch in 2025
By 2025, the construction industry is set to be transformed by sustainability, advanced technology, and innovative practices like BIM, automation, and modular building. Embracing these trends will lead to safer, smarter, and more efficient construction projects with a strong focus on environmental impact and worker well-being.
 19 March 25
19 March 25

Why Should You Use Corrosion-Resistant TMT Bar?
TMT bars, an advanced alternative to TOR steel, offer superior corrosion resistance due to their stress-free manufacturing process. They are also fire-resistant and provide excellent bonding strength with concrete, making them ideal for durable and safe construction.
 23 May 22
23 May 22

How to Prevent Corrosion in TMT Bars?
Corrosion weakens metals by reacting with environmental elements like moisture and salt. Prevent it on TMT bars by proper storage, using corrosion-resistant materials, and applying protective coatings or plating to shield the metal surface.
 21 May 22
21 May 22

How To Protect Your Window Grill From Rust?
Window grills enhance your outdoor decor but are prone to rust due to constant exposure to weather. To keep them looking new and lasting longer, regularly clean them with warm water and soap, protect them with metal-specific primer and paint, and promptly treat any rust spots by sanding and repainting. These simple steps prevent corrosion and maintain their shine.
 02 May 22
02 May 22

55 Amazing Architecture Series (INDIA)- Vol 5
India boasts remarkable architectural marvels, from the iconic marble beauty of the Taj Mahal to modern masterpieces like the Lotus Temple and Jawahar Kala Kendra. These structures blend cultural heritage with innovative design, showcasing the country’s rich history and contemporary creativity.
 22 April 22
22 April 22

7 Interesting Facts about TMT Bars You Did Not Know About
TMT bars, or Thermo-Mechanically Treated steel bars, are essential for earthquake-resistant construction due to their unique manufacturing process that creates a strong outer layer and a ductile core. They resist corrosion, can bend easily to fit designs, and provide superior strength and flexibility, making them ideal for durable and safe structures. Additionally, their heat-resistant properties help buildings withstand fire hazards, further enhancing safety.
 03 April 22
03 April 22

7 Reasons Why TMT Bars Are Preferred For Construction
TMT bars, made from high-strength steel using advanced Tempcore technology, are preferred in construction for their unique WINGRIP rib design, superior bendability, and excellent earthquake resistance. They are stronger, corrosion-resistant, fatigue-resistant, and offer great weld-ability, making them ideal for durable and innovative building projects.
 01 April 22
01 April 22

55 Amazing Architecture Series - Vol 4
Here are 11 remarkable architectural wonders from around the world, ranging from Las Vegas’s Fashion Show Mall and Luxor Hotel to London’s iconic Gherkin and Lloyd’s building. Each structure showcases unique design and cultural significance, highlighting global creativity in architecture.
 01 December 20
01 December 20

55 Amazing Architecture Series - Vol 3
Here are 11 more incredible architectural marvels from across the globe, including Macau’s towering Grand Lisboa, Spain’s modern Guggenheim Museum, India’s iconic Lotus Temple, and China’s Olympic National Stadium. Each structure showcases unique design, innovation, and cultural significance that continues to inspire.
 01 December 20
01 December 20

55 Amazing Architectures Of The World –Volume 2
Here are 11 fascinating architectural wonders from around the world, ranging from Mexico’s Calakmul Corporate Building inspired by Mayan culture to Japan’s futuristic Nakagin Capsule Tower. These unique structures showcase creativity, history, and innovation in architecture across diverse cultures and eras.
 01 December 20
01 December 20

55 Amazing Architectures of the World –Volume 1
These unique buildings—from Poland’s whimsical Crooked House and Germany’s nature-inspired Forest Spiral to the basket-shaped corporate HQ in Ohio—showcase creativity worldwide. Highlights include Salvador Dalí’s Torre Galatea museum in Spain, Canada’s modular Habitat 67, Vietnam’s fairy-tale Crazy House, and Prague’s iconic Dancing Building, each blending art, culture, and innovative design in stunning ways.
 01 December 20
01 December 20

9 Fun Facts On Steel That You Should Know
Steel is an incredibly versatile alloy with fascinating applications and history—from launching into space during nuclear tests to forming the world’s largest dental cap for an elephant. It can be endlessly recycled without losing quality, powers industries, and even expands the Eiffel Tower in summer!
 01 December 20
01 December 20

Why Were The Construction Rules Revised In India?
For years, twisted iron rods were used in Indian construction, but they corroded quickly and failed during earthquakes. The devastating 1993 Latur quake, which killed nearly 10,000 people, prompted a shift toward safer materials like TMT bars—known for their strength, ductility, and corrosion resistance. Today, TMT bars are transforming earthquake-resistant housing across India.
 01 December 20
01 December 20

How to Construct An Earthquake Resistant Building
India has faced several major earthquakes, from Latur in 1993 to Bhuj in 2001, exposing weaknesses in traditional construction. In response, strict building codes were introduced, emphasizing lightweight, flexible designs and materials like earthquake-resistant TMT bars, which offer high ductility and shock absorption. Structures built with reinforced concrete and TMT bars are now preferred for their superior resilience in seismic zones.
 01 December 20
01 December 20

15 Amazing Steel Facts That Will Surprise You
Steel, often called “white gold,” is one of the most vital construction materials in the world, known for its strength, durability, and recyclability. Around 69% of steel is recycled annually in North America—more than any other material—and it can be recycled infinitely without losing quality. Recycling one ton of steel saves 2,500 pounds of iron ore, 1,400 pounds of coal, and powers 18 million homes, making it both an engineering marvel and an environmental hero.
 22 November 19
22 November 19

15 Amazing Steel Facts That Will Surprise You
Steel plays a crucial role in modern life and the economy, with its versatility making it a staple in homes, appliances, and infrastructure. Just the recycled steel from four cars can frame a 2,000 sq. ft. house, and steel roofs last over 50 years—far outlasting traditional materials. From fire-resistant doors to termite-proof frames and recyclable tin cans, steel’s strength, durability, and sustainability truly make it the backbone of civilization.
 15 November 19
15 November 19

How to Build An Earthquake-Proof House?
If you're living in an earthquake-prone zone, building with seismic safety in mind is crucial. Use earthquake-resistant TMT bars, ensure a continuous load path, understand your seismic zone, and consider retrofitting for extra reinforcement. Proper planning and smart construction can save lives and property.
 21 December 16
21 December 16

5 Interesting Facts on the Steel & TMT Bar of India
Steel is vital to India’s economic growth, with TMT bars playing a key role in modern construction. These bars gain strength from a special Thermo-Mechanical Treatment, making them ductile, weldable, and resistant to earthquakes, heat, and corrosion. Thanks to low carbon content and advanced manufacturing, TMT bars ensure safer, stronger, and more durable structures for a rapidly developing nation.
 21 December 16
21 December 16

Why Make Earthquake-Resistant Buildings
India, being one of the most earthquake-prone countries, has revised its construction standards to improve safety in seismic zones. The use of TMT bars—known for their ductility, strength, and vibration resistance—has become mandatory in major construction projects, especially in Zones III, IV, and V. When used along with seismic isolation systems and proper design codes, TMT bars help reduce structural damage and save lives during earthquakes.
 21 December 16
21 December 16

5 Reasons Why Is Steel Preferred For Construction Purposes
The steel industry plays a vital role in India’s economic development and is crucial for the country’s industrial and infrastructural growth. Stainless steel is especially preferred in construction due to its corrosion resistance, strength, and durability. TMT bars, made from high-grade steel through advanced processes, are widely used in construction for their ability to withstand stress, natural disasters, and aging—offering long-term cost-efficiency, structural safety, and design flexibility.
 21 December 16
21 December 16

3 Insights on Earthquake-Resistant Buildings You Should Know
Natural disasters like earthquakes can be devastating, with most fatalities occurring in poorly constructed buildings—especially in developing countries. Engineering techniques such as reinforcement using TMT bars, base isolation, and bracing can significantly reduce structural damage. Additional methods like tuned mass dampers and hysteretic dampers further enhance earthquake resistance in modern buildings, improving safety and resilience.